Researchers interested in establishing collaborations may email us at ysaini@ncsu.edu
The Saini Lab is committed to sharing its scientific knowledge and technical resources with the scientific community. Over the recent years, the laboratory has assembled a variety of resources and expertise that is now available for investment in scientific collaborations. From a long list, selective examples are presented here:
1. Generation of complex multi-transgenic mice strains: We have generated over 50 different strains of mice that harbor various transgenes. We have helped faculty members in devising effective and time-efficient breeding strategies for the speedy establishment of their colonies.

The generation of novel transgenic mice strains is always paralleled with the mandatory steps where we simultaneously breed Cre recombinase mice with the reporter strains. Full characterization of these strains developed in our lab comes with the determination of whether the gene manipulation has occurred as expected.
2. High Throughput Genotyping: We have standardized high throughput PCR genotyping strategies in order to establish multi-transgenic mice colonies.
3. Immunohistochemical Staining: Our laboratory has standardized immunohistochemical staining protocols for a variety of lung disease associated proteins including MUC5B, MUC5AC, Ki-67, Ly6.B2, Major Basic Proteins, F4/80, FOXJ1, SPC, MMP12, MMP14, HMGB1, FIZZ1/RETNLA, YM1/2, HIFs, p-EGFP, IL-33, and NOS2. Selected examples from published and peer-reviewed manuscripts are shown here:
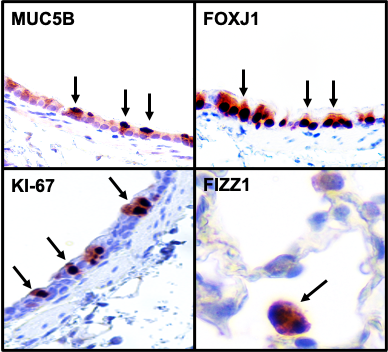

4. Histopathological Analyses by ACVP Board-certified Pathologist: Dr. Sonika Patial, our collaborator, is a board-certified anatomic pathologist with certification in digital pathology who has developed software-based digital quantitative techniques for the characterization of pathological changes in tissues. Her involvement in our research group provides us an opportunity to perform double-blinded analyses of tissues.
5. Gene expression Analyses using RNAscope: To assess in situ alterations in gene expression of selected genes, we perform RNAscope assays that allow us to analyze cell-specific gene expression changes. The following image, which was published in the Journal of Immunology in 2020, indicates distinct punctate staining of two gel-forming mucins as red and green dots.

6. Gene expression Analyses using RT-PCR and Bulk Analyses: To assess global gene expression changes, we have standardized RNA isolation protocols that allows us to extract high quality RNA (RIN/RQN >9.0-9.6) for downstream applications. Further, we are capable of performing downstream functional analysis of bulk gene expression data.

7. Flow Cytometry: Our laboratory has a dedicated 11-color flow cytometer that could be utilized to analyze leucocytic, innate lymphoid, and adaptive immune cell populations. These analyses could be performed on the bronchoalveolar lavage (BALF) cell pellets or whole lung tissue digests. We perform various magnetic bead-based enrichment of cell populations prior to flow cytometry.

8. Inflammatory Mediator Analyses: We have consistently published cytokine/chemokine analyses on BALF and blood from experimental animal models. For this purpose, we use bead-based analyses available from Millipore, BioLegend, and BioRad.
9. Toxicant and drug screening assays on Macrophages and Air Liquid Interface (ALI) cultures: Our laboratory has developed high throughput assays to screen the effects of a large panel of toxicants on macrophage activation. We have also established methodologies to develop murine ALI cultures. These culture systems could be employed to understand the interactions between epithelial cells and macrophages.

10. Exosome Purification and Characterization: We have established protocols for harvesting microvesicles from the BALF and serum. Purified exosomes could be used for downstream proteome and transcriptome analyses.

11. Surgical and Experimental Approaches: The laboratory routinely uses various techniques including oropharyngeal or intratracheal aspiration, oral gavaging, bone marrow chimera generation, intravenous and intraperitoneal injections, bronchoalveolar lavage, cytospin preparation, cecal ligation and puncture induced sepsis, and bone marrow-derived primary cell cultures.
12. Invasive Lung function test: We have acquired capabilities to assess lung compliance and resistance measurements in the rodents. This allows us to perform pulmonary lung function tests and determine the extent of lung pathology.

13. Special Stains: Our laboratory has standardized several special staining methods relevant to lung diseases including TUNEL, Hydroxyprobe, Reactive oxygen species, Masson’s Trichrome, Picrosirius red, and AB-PAS. Selected examples from published and peer-reviewed manuscripts are shown here:

Training Opportunities
Funded Graduate Fellowships: positions are available for motivated, research career-oriented undergraduate, graduate (master’s as well as PhD). Interested candidates may email their CVs to ysaini@ncsu.edu
Summer Fellowships for DVM Students: One full-time paid summer fellowship is available to DVM professionals. Fellows are expected to contribute at least 8 hours per day during the tenure of fellowship. Fellow will be working full time to initiate and complete a full-length research project. It is expected that student present and publish their research work as a lead author. Interested candidates may email their CVs to ysaini@ncsu.edu